How Chemistry Simplifies the Ironing Process
Written on
Chapter 1: The Science Behind Wrinkles
Ironing is one of those chores I dread—I'd rather tackle almost anything else. As I faced a mountain of laundry, my mind wandered to why freshly washed shirts often emerge looking like a jumbled mess. Why do cotton fabrics wrinkle so easily? And what’s the secret behind “easy-iron” garments that require less effort to press?
As a scientist, I recognize the necessity of grasping the underlying principles before diving into practical tasks like ironing. Therefore, it became crucial to uncover the answers to these pressing queries before I reached for the iron and board.
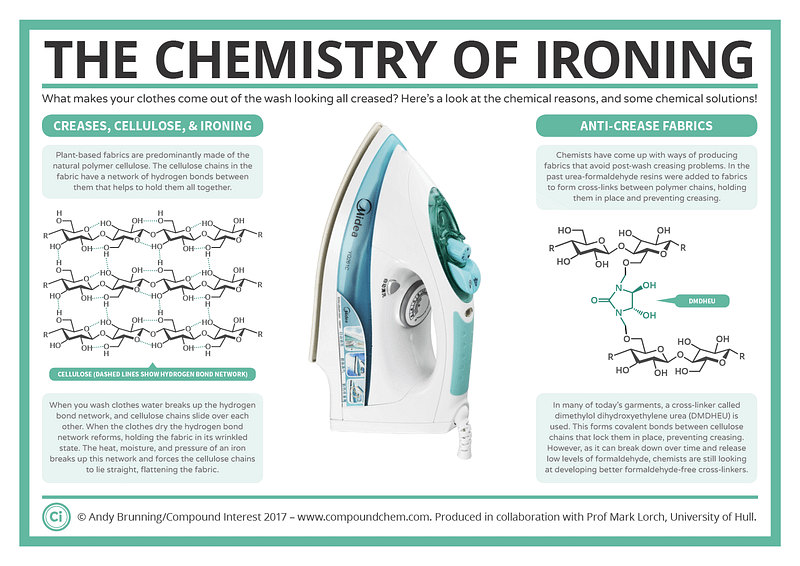
The crinkles in my clothing are primarily attributed to the chemistry of natural fibers. Fabrics such as cotton, linen, and hemp are largely composed of cellulose, a type of polymer formed from long chains of glucose molecules. Each glucose unit is “sticky” because it can bond with neighboring cellulose molecules through hydrogen bonds. While these bonds are individually weak, together they create a robust network that lends strength to the fabric.
These hydrogen bonds are quite dynamic, constantly breaking and reforming. Consequently, garments adopt the shape in which they are left. This isn't an issue if I promptly hang freshly ironed shirts. However, when I toss them into a heap—my makeshift "floordrobe"—the bonds break and reestablish, allowing the fabric to retain its new, crumpled form.
Just Add Water
Things escalate when water is introduced (like during washing). Water molecules disrupt the hydrogen bonds and act as a lubricant, enabling the cellulose strands to glide past one another. Once the fabric dries, the cotton maintains its new wrinkled shape, resulting in the unsightly pile of shirts awaiting my attention.
This is where the hot, steaming iron plays a crucial role. The combination of heat and moisture swiftly breaks the hydrogen bonds. By applying pressure, I can align the cellulose molecules parallel to each other, effectively smoothing the fabric.
But what if I want to skip ironing altogether? The wrinkled look is an option I can occasionally pull off as an academic. However, there are times when a pressed shirt is necessary. An age-old solution is to starch my clothes to keep them wrinkle-free. Starch, also a polymer made from glucose, can form similar sticky hydrogen bonds.
Unlike cellulose, starch is a branched polymer. When applied to cellulose, it acts like a framework, holding the cellulose molecules in place. The downside is that starch creates a somewhat stiff appearance and is water-soluble, meaning it washes out easily. Ultimately, this doesn't alleviate my workload; I still need to iron and reapply starch.
What I truly need is a more permanent solution. This is where easy-iron clothing comes in. Initially, formaldehyde was used to create permanent bonds between cellulose molecules, reducing wrinkling. Nowadays, formaldehyde has been replaced by more user-friendly cross-linkers like dimethyloldihydroxyethyleneurea. Although wrinkle-resistant shirts are convenient, they often have a slightly synthetic feel and can release small amounts of formaldehyde, which may irritate the skin.
The pile of laundry still looms before me, but at least I now understand the science of ironing. It looks like it’s time to tackle the practical side—or perhaps I’ll embrace the crumpled aesthetic and call myself a theoretical ironist.
Chapter 2: Practical Tips for Effective Ironing
In this video, "Chemistry of Ironing," explore how the principles of chemistry can transform your ironing experience, making it easier and more effective.
The second video, "5 Tips Guaranteed to Save Time and Improve Quality Ironing," provides practical advice to enhance your ironing skills and save precious time.