Understanding the Impact of Mirror-Reflected Molecules on Life
Written on
Chapter 1: The Basics of Molecular Structures
Everything in our environment is composed of atoms that bond to form molecules. The specific types of atoms and their spatial arrangement can alter the properties and behaviors of these molecules. Interestingly, two molecules made up of the same atoms can exhibit entirely different characteristics based solely on their three-dimensional structure. This phenomenon creates molecules that are mirror images of one another, which can significantly affect various aspects of life, from the fragrance of fruits to medical outcomes.
To illustrate the concept of molecular mirror images, consider our hands. The left and right hands are mirror reflections, yet they cannot be superimposed on one another.
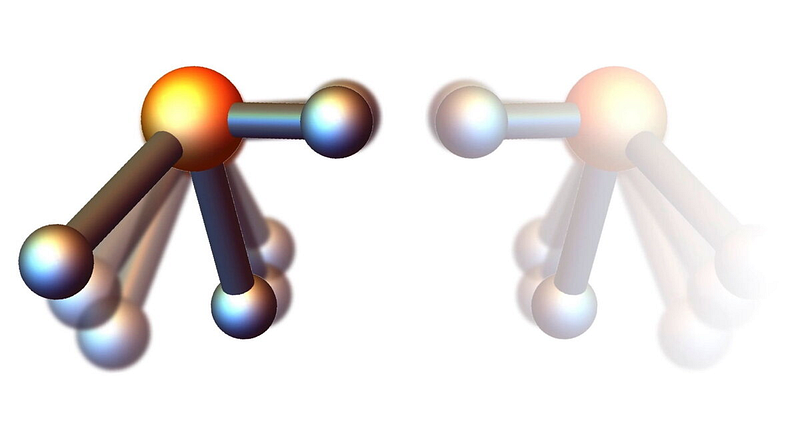
Similar to our hands, certain molecules can also be mirror images. These pairs are referred to as enantiomers. Typically, one of the enantiomers is designated with the letter R, while its counterpart is labeled S, although L and D can also be used in some cases.
Section 1.1: The Significance of Enantiomers
Let’s explore how similarly structured molecules can differ drastically in their functions and the impact these differences have on our lives. Despite being mirror images, enantiomers can trigger vastly different responses within the human body.
The refreshing aroma of citrus fruits often masks the underlying chemical complexities. Remarkably, there is one molecule responsible for the scents of both lemons and oranges. The molecule R-limonene is what gives oranges their characteristic smell, whereas S-limonene is responsible for the scent of lemons.

Thus, the next time you peel an orange, remember that its citrus fragrance is attributed to R-limonene. Conversely, the lemon scent in household cleaning products likely comes from the enantiomer S-limonene.
Subsection 1.1.1: The Aroma Connection
The link between mint leaves and caraway seeds may be less apparent. S-carvone produces the spicy aroma of caraway, while R-carvone is responsible for the cool scent of mint.
Enantiomers also play critical roles in medicine. There are numerous instances where one enantiomer of a compound serves as the active ingredient (the component that provides the desired therapeutic effect), while its mirror image does not affect the body at all. In more severe cases, the opposing enantiomer can produce harmful side effects.
For example, S-ibuprofen is the active enantiomer that alleviates pain, while R-ibuprofen, although present, has no effect. In this scenario, complete removal of the non-active enantiomer from the medication is unnecessary. However, this is not the case for the pain reliever naproxen; S-naproxen alleviates back pain, while R-naproxen can be dangerous as a liver toxin.

Chapter 2: The Thalidomide Tragedy
The first video explains how to determine whether mirror images can be superimposed, shedding light on the complexities of molecular symmetry.
The second video discusses symmetry operations, including reflections and mirror images, with practical examples.
One of the most devastating instances involving drug enantiomers was the thalidomide case. In the late 1950s, thalidomide was prescribed in Germany as a sedative, successfully treating morning sickness in pregnant women. While R-thalidomide provided the intended effects, S-thalidomide had teratogenic consequences, leading to thousands of children being born with serious limb deformities and short life expectancies. Tragically, even administering just the R-enantiomer would not have prevented these outcomes, as the body has the capacity to convert R-thalidomide into S-thalidomide. This incident prompted heightened scrutiny of enantiomers in medicine, resulting in stricter drug regulations across the globe.
While some enantiomer combinations may yield distinctive scents, others pose serious health risks. Regardless, it is remarkable how significantly the arrangement of atoms within a molecule can influence both our sensory experiences and our health.